The PLASMABIOTECH TUBES
Swiss Plasmabiotech PRP tube is certified class IIa medical device approved by Swiss Medic for the separation and collection of plasma from blood.
The company Swiss Plasmabiotech GmbH, with the coming introduction of a Medical Devices Equipment product range, has to strictly attain to the European Directive for Medical Devices 93/42/CEE – recognized in Switzerland under the Legislative Decree nr. 46 dated 24/02/97 and subsequent amendment Directive 2007/47/CE recognized under the Legislative Decree nr. 37 dated 25/01/2010 – which imposes to guarantee the traceability of the Medical devices, for a proper after-sale service
Swiss Plasmabiotech GmbH, operating in a Quality System according to the UNI 1CEI EN ISO 13485 : 2016 norms and to the above mentioned Legislative decree, has to make certain the traceability of the Medical Devices sold by your Depot is duly guaranteed.
Read More


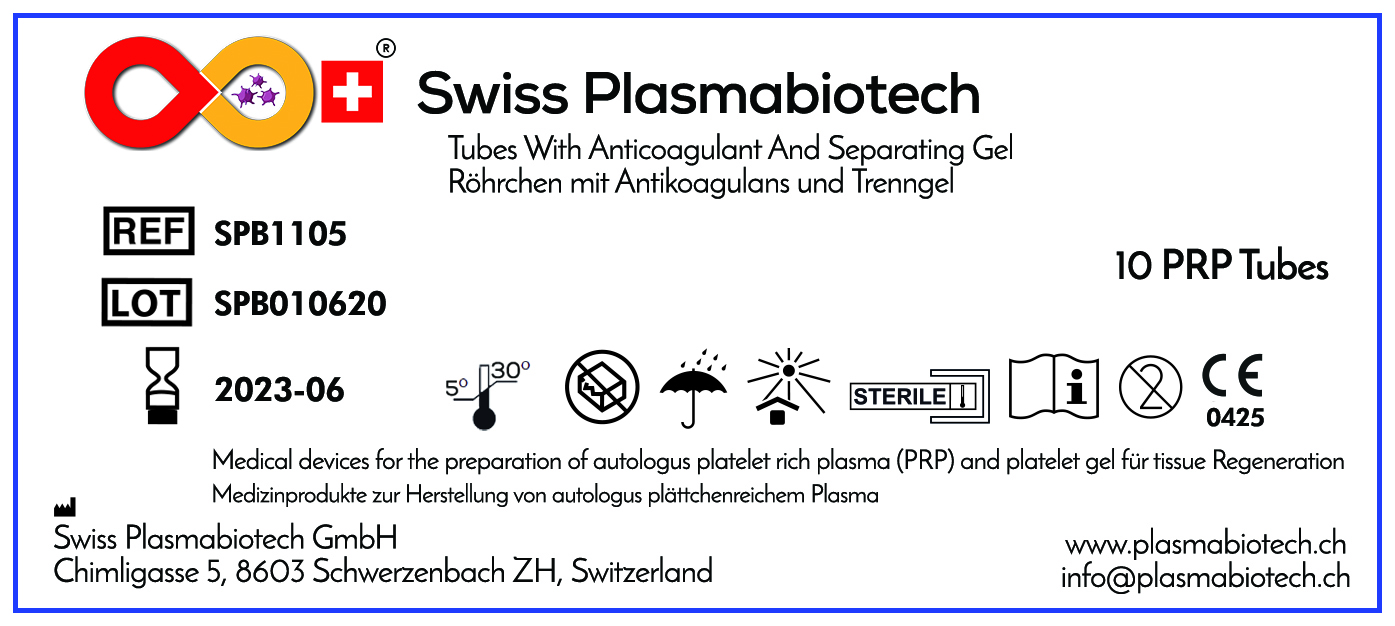
Product Values
For participative improvement of the technology knowledge, reporting on the website www.plasmabiotech.ch is recommended.
SPB PRP volume per tube
Optimal platelet recovery
Red Blood Cell Depletion
Clinical Data of
SPB PRP Tube
Source: Unilabs Switzerland, Platelet count test dated on 01/14/2021